Structural characterisation of Oligomers and fibril assemblies
α-Synuclein Oligomers
α-synuclein isa 140-residue intrinsically disordered protein (IDP) with a tendency to form aggregates and causing a group of neurodegenerative diseases known as synucleinopathies. It includes Parkinson’s disease (PD), Dementia with Lewy bodies (DLB), multiple systems atrophy (MSA) and pure autonomic failure (PAF). All these diseases have deposition of α-synuclein but have different symptoms and pathology and accumulation at different parts of the brain.The relative role of oligomers versus fibrils in synucleinopathiesis still debatable. We have been trying to understand the structure-toxicity relationship in α-synuclein oligomers and fibrils in collaboration with the group of Samir Maji at IIT, Powai. Samir’s group works on identifying new oligomers and fibrils and studying their toxicity, in vitro,at near-physiological conditions and in vivo. We primarily use solid-state NMR and other biophysical techniques to structurally characterize these aggregates and identify the structural difference between different oligomers and fibrils, in turn, improving our understanding of the structure-toxicity paradigm.
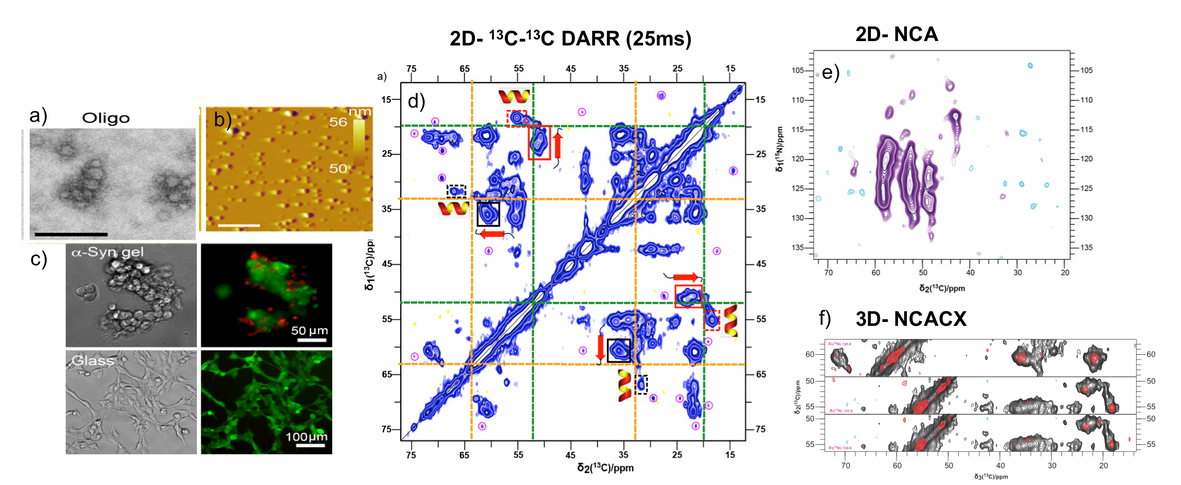
a) TEM & b) AFM picture of α-synuclein Oligomers c) Human Neuroblastoma cells in presence and absence of α-synuclein Gels d) e) and f) different solid-state NMR spectra of α-syn Gel samples characterizing the structural propensities for different residues present in the oligomers.
p53
p53 is a transcription factor that inhibits genes required for cell cycle progression and activates genes leading to apoptosis. The function of p53 is to prevent the cell from becoming cancerous by controlling its cell division. Hence, it is called “guardian of the genome”. Thus, mutations in p53 can lead to loss of function and progress of cancer in about 50% of reported human cancers. The aggregates of p53 have been observed in malignant cancers. Cells models have been used to demonstrate the loss of function and onset of tumors during p53 amyloid formation. In general, amyloids are implicated in various deadly diseases. However, p53 amyloids appear to be non-toxic but possess a high seeding capacity. This indicates that p53, once available in minute quantity inside the cell, can recruit native p53 and amplify the formation of amyloids. p53 is believed to sequester not only p53 molecules but also recruit other tumor suppressor proteins such as p63 and p73 to participate in amyloid formation. Like prions, the aggregated p53 transmits from one cell to the other, thereby turning healthy cells into a cancerous cell. We are interested in the structural characterization and structural differentiation of amyloids formed by the wt- and the mutant p53 DNA binding domain (DBD).
This project is also in collaboration with Samir Maji at IIT, Powai.
Relevant Publications:
- Rakesh Kumar, Subhadeep Das, Saroj K Rout, Saayak Halder, Ganesh M. Mohite, Narendra Nath Jha, Surabhi Mehra, Vipin Agarwal, and Samir K Maji, Neurotoxic oligomers and fibrils trapped in a gel-like state of α-synuclein assemblies. Angewandte Chemie International Edition, vol. 57, issue 19, 5262-5266 (2018)
- , , , , , , , , , , ,
